Welcome to Our Company
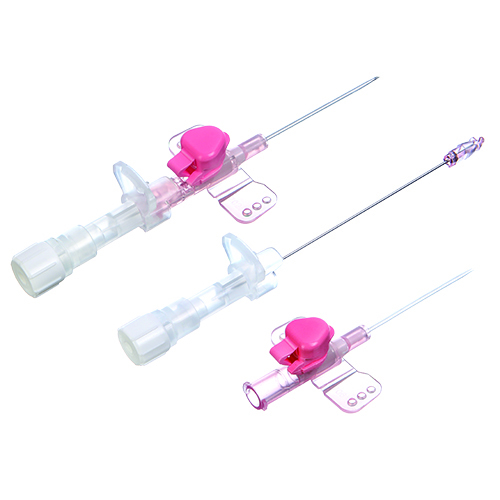
Safety Intra Venous IV Cannula
Product Details:
- Shelf Life 5 years
- Accuracy High insertion and flow accuracy
- Measurement Range Available in various Gauge sizes (14G-24G)
- Function Venous Access with Enhanced Safety
- Storage Instructions Store in a cool, dry place, Away from direct sunlight
- Usage Type Single Use/Disposable
- Features Needlestick Injury Prevention, Easy Penetration, Kink Resistant Catheter
- Click to view more
X
Safety Intra Venous IV Cannula Price And Quantity
- 5000 Piece
Safety Intra Venous IV Cannula Product Specifications
- Intravenous Access for Fluid & Drug Administration
- Lightweight (~2-5 g)
- IV Cannula
- Color-coded hub according to Gauge
- Available in various Gauge sizes (14G-24G)
- No
- 5 years
- Custom as per Gauge Size
- High insertion and flow accuracy
- Manual Insertion
- Needlestick Injury Prevention, Easy Penetration, Kink Resistant Catheter
- Other
- Silent
- Yes
- Store in a cool, dry place, Away from direct sunlight
- Single Use/Disposable
- Yes
- Safety Shielded Needle
- Venous Access with Enhanced Safety
- Sterile, Single-Use
Safety Intra Venous IV Cannula Trade Information
- Nhavasheva Port, Mundra Port, Hajira Port.
- Letter of Credit (L/C), Western Union, Paypal, Letter of Credit at Sight (Sight L/C), Cash Against Delivery (CAD), Telegraphic Transfer (T/T), Cash in Advance (CID), Cash Advance (CA)
- 500000 Piece Per Week
- 5 Days
- Yes
- Contact us for information regarding our sample policy
- Box Quantity- 50 Master Box Quantity- 1000
- Western Europe, Australia, Eastern Europe, Middle East, Central America, South America, Asia, North America, Africa
- Assam, Dadra and Nagar Haveli, Chandigarh, Himachal Pradesh, Nagaland, Uttarakhand, Daman and Diu, Lakshadweep, South India, East India, West India, Andaman and Nicobar Islands, Andhra Pradesh, Arunachal Pradesh, Delhi, Gujarat, Goa, Jammu and Kashmir, Jharkhand, Karnataka, Kerala, Maharashtra, Mizoram, Meghalaya, Manipur, Punjab, Rajasthan, Sikkim, Tamil Nadu, Tripura, West Bengal, Haryana, Pondicherry, Madhya Pradesh, North India, Bihar, Telangana, Central India, Odisha, Chhattisgarh, Uttar Pradesh, All India
- ISO/CE Certified
Product Description
Special Features
Safety mechanism Smooth edged safety needle Prevents needle-stick injuries and blood splash. Size Length 18 G 45 mm 20 G 32 mm 22 G 25 mm 24 G 19 m
Venesafe
- Specially designed innovative safety cannula.
- The cannula protects the healthcare provider from the risks associated with accidental needle stick injuries.
- It allows caregivers safe and easy I.V. access.
- Special double tapered and Kink resistant FEP-catheter.
- Unique mechanism on the tip which immediately covers the sharp tip of the needle upon withdrawal.
- Self-activating safety mechanism prevents the incidences of needle stick injuries and blood splash.
- Robust needle tip protection.
- Triple faceted needle tip for improved patient comfort and reduced incidences of complications like phlebitis.
- Luer lock allows universal attachment to all standard devices.
- Sterile, Individually packed.
Enhanced Patient and Clinician Safety
Designed with an automated or passive safety protective shield, this IV cannula significantly reduces the risk of needlestick injuries. The kink-resistant catheter, combined with high insertion accuracy, ensures both patient and healthcare provider safety throughout intravenous therapy.
Optimized for Diverse Clinical Needs
Available in multiple needle sizes (14G-24G) and catheter lengths (19-45 mm), the cannula offers flexibility for a variety of intravenous access requirements. Its color-coded, transparent hub and radiopaque strip enable easy identification and precise placement under imaging.
Convenience and Compliance in Every Detail
Individually blister packed, each cannula is sterile and latex-free, adhering to strict ISO and Indian standards. Its lightweight, portable design supports single-use applications, suitable for a broad range of healthcare settings, ensuring reliability and peace of mind for both users and patients.
FAQ's of Safety Intra Venous IV Cannula:
Q: How does the safety shield on the IV cannula help prevent needlestick injuries?
A: The IV cannula features an automated or passive safety protective shield that covers the needle immediately after use, minimizing exposure and reducing the risk of accidental needlestick injuries, thereby enhancing clinician safety during insertion and disposal.Q: What are the available size options for the IV cannula and how do I select the right gauge?
A: The cannula comes in needle sizes ranging from 14G to 24G, each with a specific catheter length from 19 mm to 45 mm. Selection depends on patient vein size and the required fluid flow rate, with color-coded hubs aiding in quick identification.Q: When should this IV cannula be used, and is it suitable for all patients?
A: This IV cannula is intended for single-use intravenous access in fluid and drug administration across a range of patient profiles. Its latex-free and sterile properties make it suitable for most patients, including those with latex sensitivities.Q: Where should the IV cannula be stored before use?
A: Store the cannula in a cool, dry place away from direct sunlight to preserve sterility and product integrity. The packaging ensures the product remains uncontaminated until needed.Q: What is the process for inserting this cannula, and what features assist with accurate placement?
A: Manual insertion is performed using the bevelled, atraumatic tip for smooth vein penetration. The transparent, ergonomic hub with clear identification markings, along with a radiopaque strip, assists in proper placement and verification under X-ray if needed.Q: How does the radiopaque strip benefit clinical procedures?
A: The radiopaque strip in the catheter permits straightforward visualization on X-rays, enabling clinicians to confirm correct placement and reduce complications from malpositioned cannulas.Q: What is the shelf life of the IV cannula, and can it be reused?
A: Each IV cannula has a 5-year shelf life and is designed for single use only. Reuse is strictly discouraged to maintain patient safety and prevent infection.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email




 Call Me Free
Call Me Free
