Welcome to Our Company

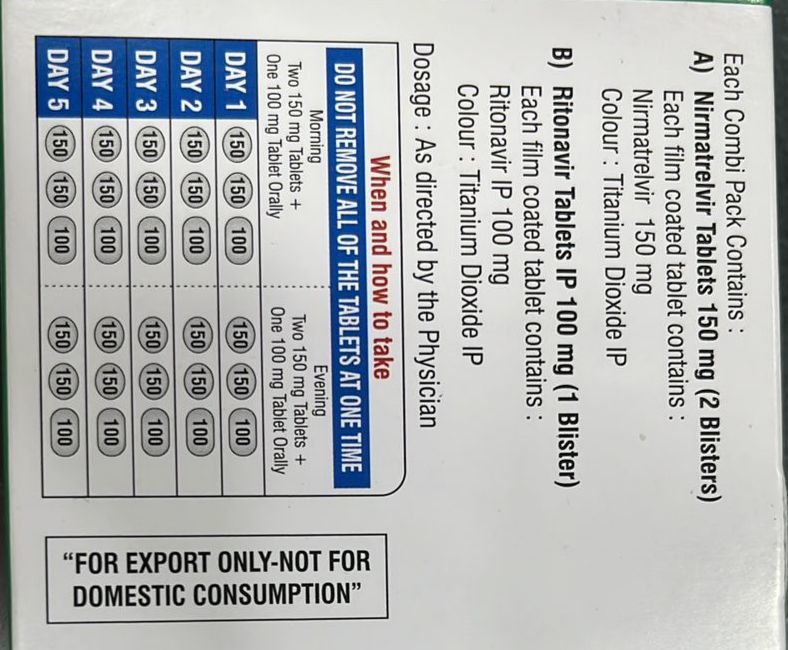
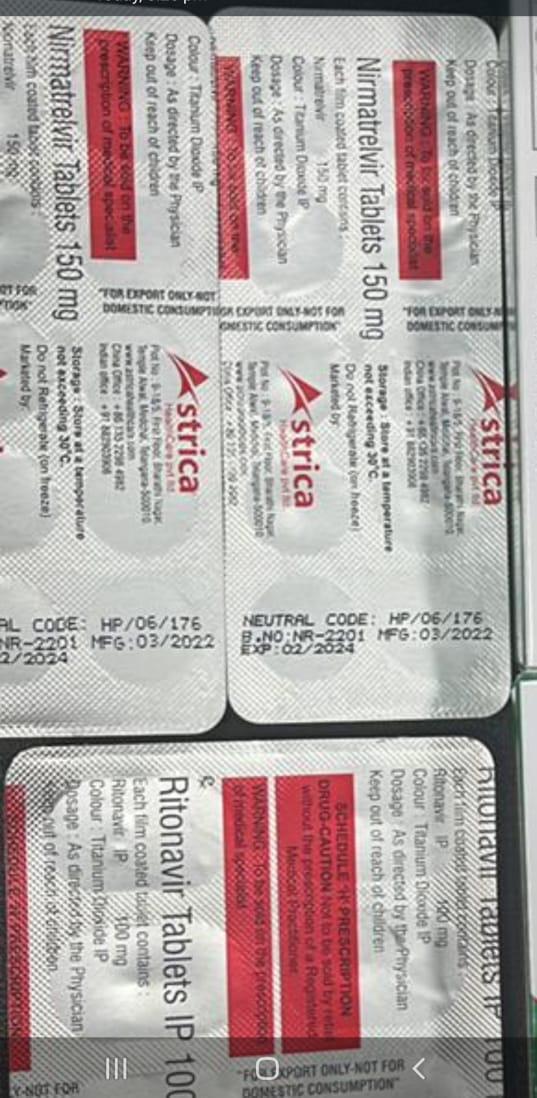
Nirmatrelvir 150mg Tablets Ritonavir 100mg Tablet
Product Details:
- Drug Type General Medicines
- Ingredients Nirmatrelvir Tablets 150 mg & Ritonavir Tablets IP 100 mg
- Physical Form Tablets
- Function Other
- Recommended For Treatment: COVID-19
- Dosage As directed by doctor
- Dosage Guidelines As directed by physician
- Click to view more
X
Nirmatrelvir 150mg Tablets Ritonavir 100mg Tablet Price And Quantity
- 10 Pack
Nirmatrelvir 150mg Tablets Ritonavir 100mg Tablet Product Specifications
- 2*10 & 1*10 Boxes
- Suitable For All, Adults
- Other
- As directed by physician
- As directed by doctor
- Treatment: COVID-19
- General Medicines
- Nirmatrelvir Tablets 150 mg & Ritonavir Tablets IP 100 mg
- Tablets
Nirmatrelvir 150mg Tablets Ritonavir 100mg Tablet Trade Information
- Mumbai International Airport or By sea
- Letter of Credit (L/C), Paypal, Letter of Credit at Sight (Sight L/C), Cash Against Delivery (CAD), Cash in Advance (CID), Cash Advance (CA)
- 500 Pack Per Month
- 7 Days
- Yes
- If order is confirmed we will reimburse the sample cost
- 2*10 Nirmatrelvir Tablets 150 mg 1*10 Ritonavir Tablets IP 100 mg
- Australia, North America, South America, Eastern Europe, Western Europe, Middle East, Central America, Asia, Africa
- Dadra and Nagar Haveli, Himachal Pradesh, Andaman and Nicobar Islands, Nagaland, Pondicherry, Uttarakhand, Daman and Diu, Lakshadweep, South India, North India, East India, Assam, Arunachal Pradesh, Bihar, Chandigarh, Goa, Haryana, Jammu and Kashmir, Jharkhand, Karnataka, Madhya Pradesh, Maharashtra, Mizoram, Meghalaya, Manipur, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, West Bengal, Kerala, Delhi, Gujarat, Punjab, Andhra Pradesh, Central India, Odisha, West India, Chhattisgarh, Uttar Pradesh, All India
- GMP/WHOGMP/NAFDAC/COA/COPP/Manufacturing License/Free sale certificate/Dossier.
Product Description
The combination of nirmatrelvir tablets and ritonavir tablets is a product that the FDA is allowing to be given for emergency use to treat COVID-19. It is used by people 12 years of age and older who have recently tested positive for coronavirus, have had mild to moderate symptoms for no more than 5 days and are not hospitalized.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email






 Call Me Free
Call Me Free
